Summary
A recent Yale study highlights immune system dysregulation in Post-Vaccine Syndrome (PVS) patients, characterized by chronic inflammation, B cell abnormalities, exhausted T cells, viral reactivation (EBV), and emerging autoantibodies. Managing PVS requires a multi-pronged approach focusing on reducing inflammation, restoring immune balance, clearing persistent spike protein, preventing viral reactivation, and addressing clotting risks. Key strategies include Low-Dose Naltrexone (LDN), omega-3 fatty acids, vitamin D, probiotics, short-term corticosteroids, antivirals, fibrinolytics, and, in severe cases, advanced therapies like plasmapheresis or IVIG. Symptom management targets neuropathy, dysautonomia, cognitive dysfunction, and mitochondrial support.
Physicians are advised to begin with lifestyle modifications, herbal therapies, and high-quality supplements before introducing pharmaceutical interventions. Regular monitoring for autoimmune markers and clotting factors is essential, and treatment plans should be tailored to each patient's immune profile and symptoms.
A recent Yale study (preprint) provided a picture of the immune system in post vaccine syndrome (PVS) patients. I have already recorded a video presenting this picture. Sadly, this picture is evidence for an overstimulated immune system that is then dysregulated due to intense workload. The video is linked below. Many kool beens asked about the management approach for this condition. Here I am presenting strategies for a physician to consider when managing a patient with this immune profile.
My video review of the study:
Management approaches
Managing PVS requires a multi-pronged approach that blends elements from chronic inflammatory disease management, viral reactivation suppression, and post-viral syndrome care. This is a rapidly evolving field, and tailoring treatment to the individual’s immune profile and symptom set is key. By addressing inflammation, supporting immune regulation, promoting antigen clearance, and correcting B cell dysregulation, we aim to improve patient quality of life while preserving essential immune function.
Medical Disclaimer:
This document is intended for use by healthcare professionals as a reference protocol. It is not intended for self-medication or self-management by patients. All interventions should be carefully considered, selected, and tailored by a qualified healthcare provider based on the individual’s laboratory findings, clinical presentation, and overall health profile.
Dear clinicians, hope these strategies help your patients. Don’t start everything in one go. Lean towards starting the management with lifestyle, herbal, and good quality supplements. LDN should be added as soon as possible. A short term steroid intervention will help unmask if the organ damage has occurred or the inflammation is causing the symptoms.
A summary of the immune picture observed in the study:
Key Features of Dysregulated Immunity in PVS
- B Cells:
- Increased Unswitched Memory B Cells (IgD+, CD27+).
- Reduced Double Negative B Cells (DN B cells IgD-, CD27-).
- T Cells:
- Reduced Memory CD4+ T Cells (Th1 and Th2).
- Increased Exhausted CD8+ T Cells (PD-1+, TIM3+).
- Increased TNF-α Producing CD8+ T Cells.
- Innate Immune Cells:
- Increased Non-Classical Monocytes (CD14 low, CD16 high).
- Reduced Type 2 Conventional Dendritic Cells (cDC2).
- Cytokines:
- Altered IL-7, IL-21 (B and T cell support).
- Chronic inflammatory environment with dysregulated TNF-α and IL-6 responses.
- Other Features:
- Persistent circulating spike protein.
- Evidence of EBV reactivation.
Emerging autoantibodies (anti-nucleosome IgM, anti-AQP4 IgA).
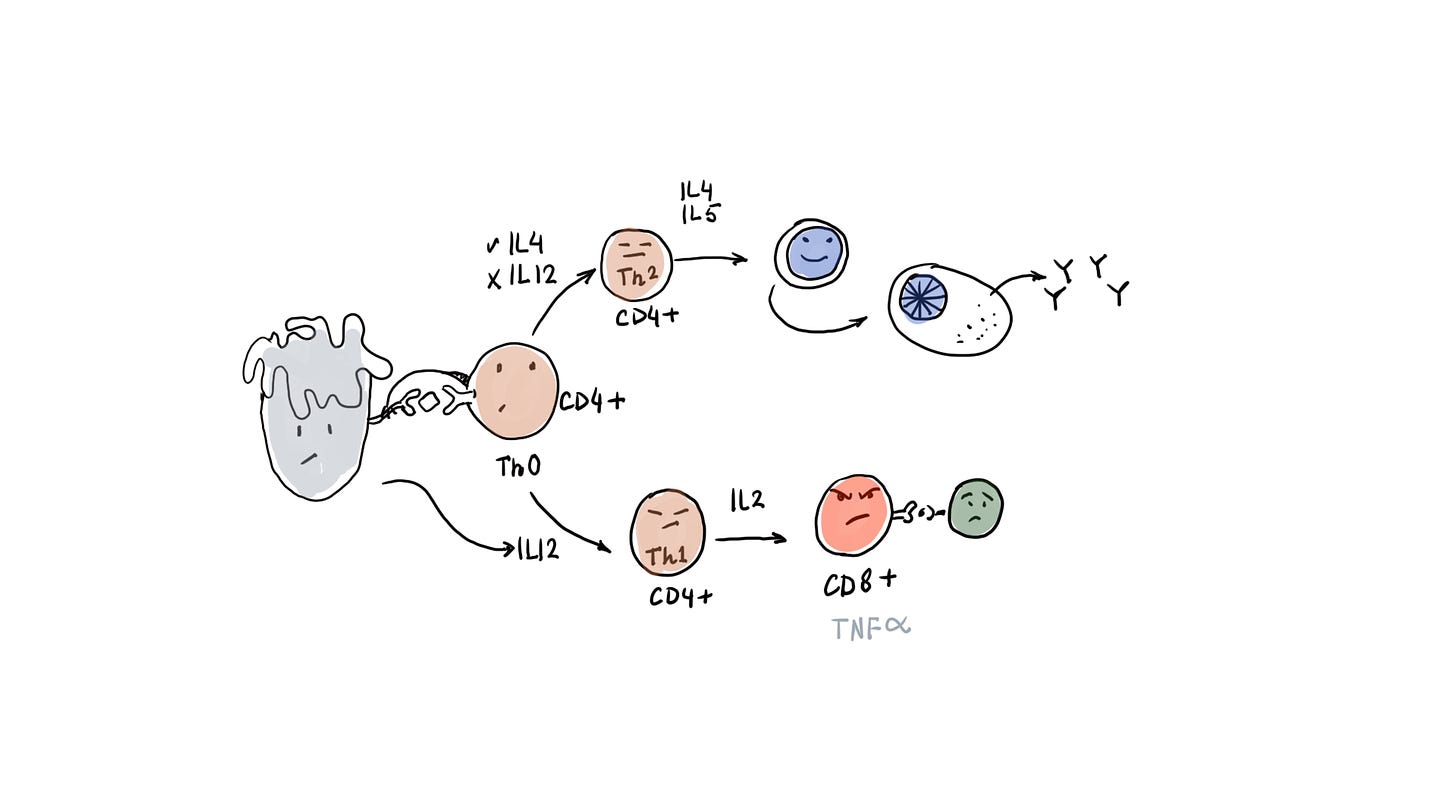
To understand this diagram please watch the initial part of the video linked above.
Management
Section 1 – Key Objectives of Management
- Reduce inappropriate inflammation.
- Restore immune regulatory balance (increase Treg function, reduce inflammatory CD8+ T cells).
- Support clearance of persistent antigens (spike protein and immune complexes).
- Prevent viral reactivation (especially EBV).
- Address emerging autoimmune features.
- Address B cell dysregulation, including supporting class switching and improving B cell maturation.
- Rebalance innate immune function (normalize non-classical monocyte populations and support cDC2 dendritic cell recovery).
- Provide targeted symptom management.
Section 2 – Inflammation Modulation Without Full Immunosuppression
Low-Dose Immunomodulators
- Low-Dose Naltrexone (LDN): Modulates monocyte and microglial activation, reduces TNFα.
- Mast Cell Stabilizers: Cromolyn sodium, ketotifen, H1/H2 blockers (loratadine, famotidine).
Short-Term Anti-Inflammatory Approaches (Severe Cases)
- Short pulse of low-dose corticosteroids (5-10 mg prednisone) in cases of significant inflammatory flares.
- Avoid prolonged use unless clear autoimmune evolution.
Targeted Biologics (Experimental)
- IL-6 inhibitors (tocilizumab) in cytokine-confirmed inflammatory cases.
- TNF inhibitors (infliximab) for cases with extreme TNFα dominance (caution due to viral reactivation risk).
Section 3 – Restoring Regulatory Immune Function
Treg Enhancement
- Omega-3 Fatty Acids (DHA, EPA): Shifts immune response toward regulatory phenotype.
- Vitamin D Optimization: Supports Treg generation and immune homeostasis.
- Specific Probiotic Strains: Certain Clostridia clusters and Bifidobacterium species induce Treg differentiation and function.
- Resveratrol: Promotes Treg expansion and enhances immunoregulatory capacity.
- NAD+ Precursors (NMN, NR): Improve T cell metabolism and may reduce T cell exhaustion.
- Low-dose IL-2 Therapy (Experimental): Selectively expands Tregs due to their high-affinity IL-2 receptors while having minimal effect on effector T cells.
Section 4 – Supporting B Cell Maturation and Class Switching
B Cell Support Strategies
- Omega-3 Fatty Acids: May improve germinal center integrity and class switching.
- Vitamin D: Supports B cell differentiation and immune balance.
- Zinc: Important for B cell development and antibody class switching.
- IL-21 Signaling Support: IL-21 is critical for class switching and B cell maturation, so monitoring and addressing overall T follicular helper (Tfh) function through restoring T cell health indirectly supports B cells.
- Targeted immunomodulation (LDN, etc.): By reducing chronic inflammation, the B cell compartment may shift back toward normal memory and class-switched phenotypes.
- Autoantibody Reduction: Normalizing B cell maturation and class switching may help reduce the production of emerging autoantibodies (anti-nucleosome IgM, anti-AQP4 IgA) by improving B cell tolerance mechanisms and reducing inappropriate antibody responses.
Section 5 – Viral Reactivation Control
Antiviral Prophylaxis (for EBV Reactivation)
- Valacyclovir or Famciclovir: Consider if EBV reactivation confirmed via serology or PCR.
- Monitor for symptom flares tied to viral reactivation events.
- Lysine supplementation – May help suppress herpesvirus replication
- Monolaurin – Has been studied for antiviral properties against enveloped viruses
- Quercetin and other flavonoids – May have both antiviral and immunomodulatory effects
- Immune-enhancing adaptogens (e.g., Astragalus) – May help support cellular immunity against viral infections
Immune Support for Viral Control: In addition to antiviral medications, addressing the reduced memory CD4+ T cells (as mentioned in our immune profile) is essential for long-term viral control, as these cells play a critical role in maintaining surveillance against latent viral reactivation.
Section 6 – Persistent Spike Protein Clearance
Enhancing Autophagy
- Spermidine, Resveratrol, Fasting: Promotes cellular autophagy to clear intracellular spike.
Extracorporeal Therapies (Severe Cases Only)
- Double Filtration Plasmapheresis (DFPP): Removes circulating spike and immune complexes (experimental, consider case-by-case).
More considerations
- Nattokinase or serrapeptase – Proteolytic enzymes that may help break down protein aggregates
- NAC (N-acetylcysteine) – Supports glutathione production which aids in protein processing and clearance
- Specific binding agents like modified citrus pectin, chitosan, or charcoal that might help bind and clear spike protein through the GI tract
Section 7 – Symptom-Specific Therapies
Dysautonomia / POTS Management
- Fludrocortisone, Midodrine, Ivabradine, Beta-blockers.
- IV Saline Infusions.
Neuropathic Pain Management
- Low-Dose Naltrexone (LDN).
- Alpha-Lipoic Acid, Gabapentin, Pregabalin.
Fatigue and Mitochondrial Support
- CoQ10, Acetyl-L-Carnitine, Nicotinamide Riboside (NR), Near Infrared Light.
Cognitive Function Support
- Phosphatidylserine, Bacopa monnieri: Support neuronal membrane integrity and cognitive function affected by neuro-inflammation.
- Lion’s Mane Mushroom: Promotes nerve growth factor and may help repair neuronal damage from inflammatory processes.
- Near Infrared Light: morning and evening walks. Near Infrared can penetrate up to four inches in the body and can pass through clothing as well.
Mitochondrial Support – Mechanistic Connections
- Anti-inflammatory Polyphenols (Curcumin, EGCG): Address the TNF-α-induced mitochondrial dysfunction and support mitochondrial biogenesis.
- PQQ (Pyrroloquinoline Quinone): Stimulates mitochondrial biogenesis to counter inflammatory damage to cellular energy systems.
Sleep Regulation
- Melatonin: Supports circadian rhythm and has additional immunomodulatory and antioxidant properties.
- Glycine: Improves sleep quality and has anti-inflammatory effects on immune cells.
- Strategize to achieve theta wave sleep states more frequently.
Section 8 – Lifestyle and Non-Pharmacologic Approaches
Anti-Inflammatory Diet
- Emphasize: Omega-3-rich foods, polyphenols (green tea, turmeric), prebiotics.
- Avoid: Processed foods, excess omega-6 oils, added sugars.
Gradual Movement and Physical Therapy
- Avoid overexertion; follow pacing strategies to prevent post-exertional symptom worsening.
Stress Reduction and Autonomic Support
- HRV Biofeedback, Meditation, Yoga, Forest bathing.
Some More Considerations
- Sleep optimization strategies, as sleep disturbances can significantly impact immune function, particularly affecting cytokine regulation and T cell function
- Time-restricted eating or intermittent fasting approaches have shown to affect immune cell function and may support autophagy
- Environmental toxin reduction strategies might be beneficial, as environmental toxins can act as immune system stressors
Section 9 – Autoimmunity Surveillance
Regular Screening for Emerging Autoimmunity
- Quarterly laboratory assessment: ANA panel, dsDNA, anti-nucleosome, anti-AQP4.
- Additional organ-specific antibodies based on clinical presentation: Thyroid antibodies (TPO, TG), tissue transglutaminase, myositis panel.
- Monitor for organ-specific symptoms indicating evolving autoimmune disease.
- Intervention thresholds: Consider more aggressive immunomodulation if antibody titers rise significantly over two consecutive assessments or when organ-specific symptoms emerge with corresponding antibody positivity.
- Specialist referral: Rheumatology or relevant specialist consultation when sustained autoantibody elevations or concerning clinical features develop.
Section 10 – Clotting Risk Management
Monitoring Parameters
- Regular assessment: D-dimer, fibrinogen, PT/INR quarterly and during inflammatory flares.
- Specialized testing: Consider SARS-CoV-2 spike protein S1 microclot analysis where available.
Antithrombotic Strategies
- Low-dose aspirin (81 mg daily) for patients with elevated D-dimer or microclot evidence.
- Low-dose DOACs (apixaban, rivaroxaban) in higher risk patients with thromboinflammatory markers.
- Antiplatelet alternatives (clopidogrel, dipyridamole) for patients with aspirin intolerance.
- Fibrinolytic support (nattokinase, lumbrokinase) (investigational, case-by-case basis).
Supportive Measures
- Hydration: Maintain optimal hydration status to reduce blood viscosity.
- Movement strategies: Regular gentle movement to prevent venous stasis.
- Compression garments: Consider for patients with evidence of venous insufficiency or pooling.
Section 11 – Advanced Immunotherapy Options (Severe/Refractory Cases)
IVIG Therapy
- Consider in patients with:
- Confirmed autoantibodies.
- Small fiber neuropathy or autonomic neuropathy.
- Clear evidence of immune dysregulation and systemic inflammation.
- Dosing strategies:
- Standard protocol: 2g/kg divided over 2-5 days
- Low-dose protocol: 0.4g/kg monthly for maintenance
- Monitoring: Complete blood count, renal function, thromboembolic markers before and during therapy
- Caution: Assess thrombotic risk before initiating IVIG.
Alternative Advanced Therapies
- Rituximab: Consider for severe B-cell mediated cases with persistent B cell abnormalities unresponsive to conventional therapy.
- Low-dose IL-2 therapy: Experimental approach for cases with demonstrated severe Treg deficiency.
- Plasmapheresis: Alternative to IVIG for antibody-mediated cases, especially with concurrent elevated inflammatory markers.
Specialist Consultation
- Immunology/Rheumatology evaluation mandatory before initiating these therapies
- Regular reassessment of risk-benefit ratio throughout treatment course
Dear clinicians, hope these strategies help your patients. Don’t start everything in one go. Lean towards starting the management with lifestyle, herbal, and good quality supplements. LDN should be added as soon as possible. A short term steroid intervention will help unmask if the organ damage has occurred or the inflammation is causing the symptoms.
Medical Disclaimer:
This document is intended for use by healthcare professionals as a reference protocol. It is not intended for self-medication or self-management by patients. All interventions should be carefully considered, selected, and tailored by a qualified healthcare provider based on the individual’s laboratory findings, clinical presentation, and overall health profile.

